Abstract
Introduction
Minimal residual disease (MRD) negativity is a strong positive prognostic factor for Multiple Myeloma (MM). Consequently, the international myeloma working group (IMWG) included MRD-negativity as a new response criterion for MM patients in 2016 (Kumar et al. Lancet Oncol. 2016). Usually, MRD assessment is based on a bone marrow (BM) sample from the iliac crest and is performed after patients reach a complete remission (CR). However, in order to avoid a painful BM aspiration, the "ultimate goal" is to use a peripheral blood (PB) based test to monitor the efficiency of treatment and assess the MRD status. In this study we have tested the suitability of circulating tumor cell (CTC) quantification as a surrogate measurement for BM MRD assessment using a highly sensitive PCR assay (sensitivity level ≤10-6). The second aim of our study was to determine the prognostic value of the level of CTCs after autologous stem cell transplantation (ASCT).
Materials and Methods
We included 66 patients who were treated within the open-label, randomized, multicenter phase III clinical trial MM5 (GMMG, EudraCT No. 2010-019173-16) and who reached a CR or suspected CR until spring 2014. BM was collected at diagnosis and at CR or suspected CR (CR N=79/115). PB was collected at diagnosis and after Velcade-containing induction therapy, ASCT, consolidation therapy, during Maintenance and at the end of the study (CR N=105/270). To evaluate the suitability of CTC quantification as surrogate measurement for MRD assessment in BM, we compared 70 BM/PB pairs (N=45 patients), which were collected simultaneously. For tumor cell quantification we used a two-step procedure of tumor cell identification via patient specific immunoglobulin heavy chain (IgH) and kappa/lambda light chain (k/λ LC) sequencing, followed by tumor cell quantification by an extreme limiting dilution approach (ELDA) PCR. In a landmark analysis starting from MRD evaluation after ASCT, progression free survival (PFS) and overall survival (OS) were analyzed to estimate the prognostic value of CTC quantification and MRD assessment in BM. Kaplan Meier plots and corresponding log-rank test p-values were generated. Cox regression was used to estimate the hazard ratio.
Results
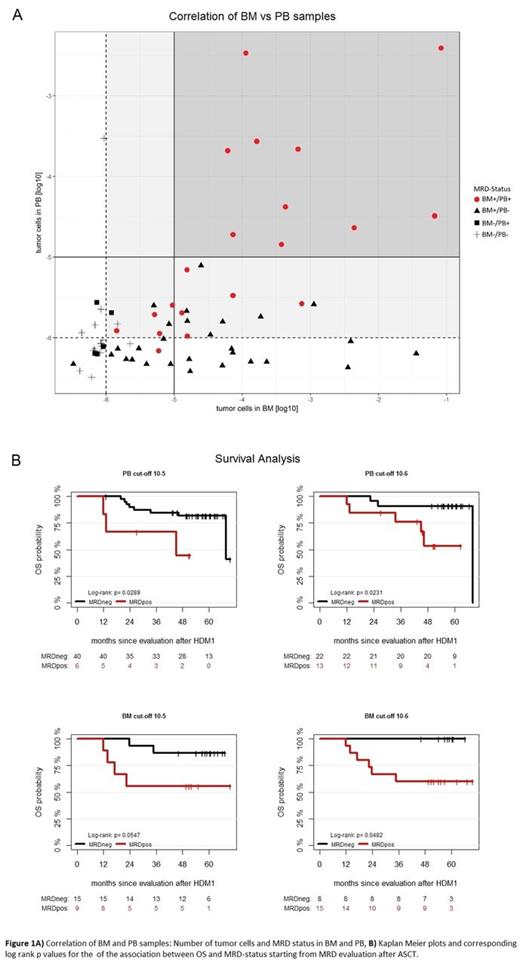
Comparing the MRD status in BM with the level of CTCs at a detection threshold of 10-6, CTCs can predict MRD-positive results in BM with high specificity: for 19 of 20 PB samples with detectable CTCs, we could also detect MM cells in the paired BM (Figure 1A). However, for 19 of 34 pairs which were negative for CTCs, MM cells were detectable in the BM. As such the specificity of a BM MRD predictor based on CTC levels was 0.88 while sensitivity was 0.5. Importantly, the sensitivity was severely impaired (0.3 only) when using a detection threshold of 10-5 for CTC quantification, emphasizing the need for highly sensitive detection methods. Next, we investigated the prognostic value of CTCs levels after ASCT. Using a detection threshold of 10-6, 13 of 35 patients presented with CTCs after ASCT. These patients had a worse OS compared to patients without CTCs after ASCT (p=0.023, Figure1B). The same holds true for BM MRD-positivity (p=0.048; 15/23 BM+ after ASCT; Figure 1B).
Conclusions
CTCs are a highly promising surrogate marker which can predict MRD-positivity in BM with a high specificity and are also associated with poor OS of MM patients. In order to avoid unnecessary BM aspirations, we propose to implement CTC quantifications into an algorithm for response analysis for MM patients until CTCs are not detectable anymore. Of note, this could be done even before the CR assessment in BM according to IMWG criteria.
Hänel: Novartis: Honoraria; Roche: Honoraria. Salwender: Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria; Honoraria and travel support: Janssen Cilag, Celgene, BMS.: Honoraria, Other: Travel support. Vogel: Janssen-Cilag Germany: Employment. Angermund: Janssen-Cilag Germany: Employment. Weisel: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Duering: Celgene: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Goldschmidt: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millenium: Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Morphosys: Research Funding; Onyx: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal